Viral Vectors for gene transfer
What is gene transfer technology?
Gene transfer is defined simply as a technique to efficiently and stably introduce foreign genes into thegenome of target cells. Genes are the basic hereditary units of all life. It is our genes that provide the blueprints necessary to produce all proteins in our bodies, and our proteins that ultimately perform every biological function. Thus, when a gene is stably introduced into a target cell the protein encoded by the gene is produced.
Gene transfer technologies were originally developed as a research tool for investigating gene expression and function. However, as new gene transfer technologies are developed and old technologies refined, the potential
applications
have expanded dramatically. Currently, there are a number of gene transfer technologies available which vary greatly in their efficiency of gene transfer and the types of cells they are capable of delivering genes into. Genes can be delivered into cells using lipid-based vectors, naked DNA, electroporation (the application of electrical charge to cells), or viruses which are the most efficient gene transfer vectors.
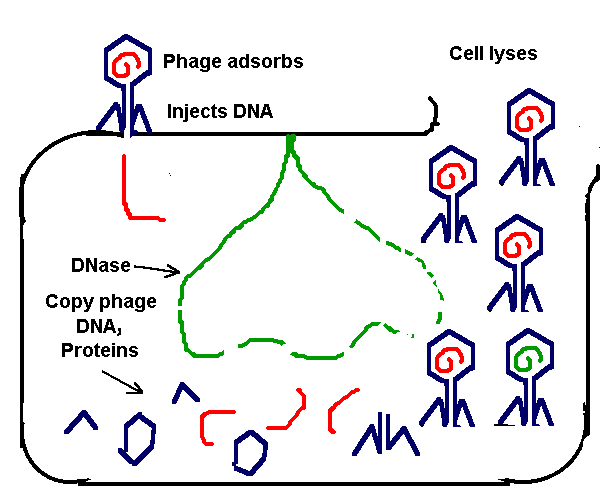
Recently, gene transfer technology has found its way into clinical applications designed to treat inherited diseases, cancer, and infectious diseases such as AIDS. When genes become altered or damaged so that the encoded protein is no longer functional, as is the case for inherited diseases and many cancers, disease development may occur. Disease development can also be the result of an acquired or infectious disease wherein a pathogenic protein or aberrant protein is produced. In either case, gene transfer technologies can be used to deliver a therapeutic gene into patients. For human clinical applications, gene transfer technologies must be designed that are capable of efficiently delivering genes into primary human cells without harming the recipient. In general, a carrier molecule known as a “vector” is used to deliver the therapeutic gene into target cells. The most commonly used vector for clinical gene transfer applications is a virus which has been genetically engineered to carry the therapeutic gene, but does not have the ability to multiply or cause disease (Figure below).
Lentiviral vectors as gene delivery systems
It is not surprising that the most efficient gene transfer technologies available today exploit the natural ability of viruses to infect and deliver genes into animal cells. Scientists have engineered viruses into gene delivery vectors or carriers that do not cause disease or multiply in infected cells. Oncoretroviruses are the most widely used viral vectors thus far because the retroviral genome inserts into the target cell’s genome following infection. As a result, retroviral vectors can permanently deliver a gene to target cells. However, the use of oncoretroviruses is limited by their ability to infect only dividing cells and by their limited success at establishing stable gene expression in humans. To circumvent these problems, alternative gene transfer technologies are being developed that are capable of delivering genes to a wide range of human cells including non-dividing cells such as neurons. One such gene transfer technology is based on lentiviruses.
Lentiviruses are a family of retroviruses known as slow viruses because symptoms do not generally appear until long after the initial infection. Like all retroviruses, lentiviruses insert their genome into the infected cell’s genome following infection resulting in stable long-term gene expression. Unique to lentiviruses is the ability to naturally infect both dividing and non-dividing cells. Thus, lentiviral-based vectors can be used to deliver genes into non-dividing human cells. This feature greatly expands the scope of potential gene transfer applications.
In developing lentiviral vectors, DNA encoding some or all of the viral genes is removed and replaced with the foreign gene. Thus, the viral vector is designed to be able to enter the cell, deliver the gene, but does not have the ability to replicate or cause disease once inside. The continued development of lentiviral vectors will be critical for the advancement of clinical gene transfer as a viable treatment option for a variety of diseases including genetic disorders and infectious diseases.
GeneCure has developed a lentiviral-based gene transfer technology
GeneCure has developed a patented platform technology of gene transfer based on a primate lentivirus, the simian immunodeficiency virus. This novel gene transfer technology has the ability to efficiently transfer genes into primary human cells without causing harm to humans. This technology will be used to develop vaccines for the prevention and treatment of human diseases.
How GeneCure’s lentiviral-based gene transfer technology works

GeneCure’s patented gene transfer technology is based on a primate lentivirus, the simian immunodeficiency virus (SIV). The company’s lentiviral gene transfer vector (SimVec) is a genetically engineered SIV genome that lacks the genes necessary for viral replication. A therapeutic gene can be introduced into the vector through standard molecular biology techniques. Virus particles encoding the therapeutic gene can be produced in a specialized cell line called a packaging cell line. Virus particles containing the therapeutic gene can then be collected and used to infect and deliver the gene to target cells. Importantly, the virus is capable of infecting and delivering the therapeutic gene to target cells including non-dividing human cells but is unable to multiply and spread to other cells. Because lentiviruses stably integrate into the target cell’s genome, the therapeutic gene can be expressed long-term and is replicated and passed on to all daughter cells during cell division.
Advantages of GeneCure’s gene transfer technology
Efficient gene delivery into primary cells
Current clinical gene transfer is hampered by the lack of effective means to deliver genes into primary human cells. The most commonly used gene transfer technologies in clinical studies are retroviral-based vectors derived from murine retroviruses. Unfortunately, these vectors have limited potential for clinical applications due to their inability to infect non-dividing cells. To address this concern, scientists have taken advantage of lentiviruses, which have the natural ability to infect non-dividing mammalian cells. Lentiviral-based vectors including those based on human immunodeficiency virus, HIV-1, have been developed to deliver genes to non-dividing human cells. However, as HIV-1 is a major human pathogen, the use of HIV-1-based gene transfer technology poses significant concerns for use in human gene transfer applications. GeneCure’s unique technology is based on the simian immunodeficiency virus (SIV), a lentivirus family member that does not cause disease in humans. Thus, GeneCure’s gene transfer technology can effectively deliver genes into human cells including non-dividing and terminally differentiated cells without the risks associated with an HIV-1-based approach.
Safety profile in primate model
In order for viral vectors to be worthy of clinical applications, they must be proven safe in animal models. The primary concern when using retroviruses for human gene transfer applications is the generation ofreplication-competent retrovirus (RCR). Studies performed in rhesus monkeys to monitor formation of replication-competent virus have validated GeneCure’s gene transfer technology as a safe delivery system for future use in clinical studies.
Stable long-term expression of gene
Because lentiviruses permanently integrate into the target cell’s genome, lentiviral vectors allow for stable long-term expression of the gene. Numerous reports demonstrate stable expression of reporter genes for greater than nine months. Additionally, unlike commonly used onco-retroviral vectors, where transcriptional silencing of the gene has been observed in numerous reports, no transcriptional silencing has been observed with lentiviral vectors. Thus, use of lentiviral vectors may overcome the challenges hindering current gene transfer technologies.
No pre-existing immunity
An important consideration when using viral vectors for clinical gene transfer applications is the presence of pre-existing immunity in the target population. Pre-existing immunity occurs when a patient has been previously exposed to the natural virus, as is common for vectors based on the adenovirus (cause of the common cold) and canarypox virus (a harmless relative of smallpox). Previous exposure the virus prepares the patient’s body to quickly mount an immune response should it encounter the virus again. As a result, use of these viral vectors in patients with pre-existing immunity may dampen the effectiveness of the vector due to unwanted immune responses directed at the vector itself. Pre-existing immunity can limit both the ability of the vector to efficiently deliver the gene to target cells as well as limit the duration of gene expression due to immune-mediated destruction of infected cells. In addition, pre-existing immunity can reduce the efficiency of vector re-administration, limiting the usefulness of boosting or re-immunization with the same vector.
GeneCure’s gene transfer technology is based on the simian immunodeficiency virus (SIV), which is not a human pathogen. Accordingly, this technology can be used in clinical gene transfer applications without the problems associated with pre-existing immunity. This includes the ability to re-administer the viral vector to patients as necessary.
Broad host range
GeneCure’s unique gene transfer technology allows production of lentiviral particles which have incorporated the vesicular stomatitis virus G protein (VSV-G) into the viral envelopes. Incorporation of VSV-G envelope protein permits the viral particles to infect a broad range of mammalian and non-mammalian host cells including human, primate, mouse, hamster, and fish cells. This technology greatly expands the applications of gene transfer technology to include studies using cell types which are resistant to infection with other vectors and to non-mammalian studies.
Stable high titer production of viral particles
Many viral-vector mediated gene transfer applications, particularly clinical applications, require a large quantity of viral vector. As a result, viral vectors must be produced at high titers and must be able to withstand further concentration. GeneCure’s unique gene transfer technology allows production of lentiviral particles which have incorporated the vesicular stomatitis virus G protein (VSV-G) into the viral envelopes. Incorporation of VSV-G into viral particles greatly increases the amount and stability of vector produced. Viral vector can be consistently produced at a biological titer of 108 Transducting Units (TU)/ml and can be concentrated to 1011 TU/ml by ultracentrifugation.
Potential applications of GeneCure’s gene transfer technology
GeneCure’s SimVec gene transfer technology has been designed as a multi-use gene product platform. Because the technology can be used to introduce any foreign gene into virtually any cell type, the potential applications are endless.
Clinical gene transfer applications
The most obvious and potentially most beneficial application of GeneCure’s gene transfer technology is the development of gene-based therapeutics to combat human disease. The lentiviral-based gene transfer technology can efficiently and stably deliver virtually any therapeutic gene safely into humanprimary cells. Thus, this technology can be used in clinical gene transfer applications designed to treat any number of human diseases including inherited disease, acquired diseases such as cancer, and infectious diseases. GeneCure will license its lentiviral-based gene transfer technology rights to other organizations developing a wide range of clinical gene transfer products.
Vaccine Development
In addition, GeneCure’s gene transfer technology will allowed for the development of innovative vaccine designs. The technology can be used to generate either preventive or therapeutic vaccines against infectious diseases such as AIDS, SARS, malaria, tuberculosis, hepatitis A, B and C viruses, influenza virus, La Crosse virus, and Ebola virus. GeneCure will be developing its own proprietary gene-based vaccines for the treatment of many human diseases including HIV, Hepatitis B, Hepatitis C, and SARS.
Production of transgenic animals
A transgenic animal is an organism that has had DNA introduced into one or more of its cells artificially. Transgenic animals are produced by introducing the DNA of interest into embryonic stem (ES) cells. ES cells containing the DNA insert are then injected into the embryo of a pregnant female; the embryo is implanted into the female’s uterus and allowed to develop. As the animal develops, every cell derived from the modified ES cells contains the specific DNA insert. In this way, whole animals can be produced with the desired modified DNA. Transgenic animals are powerful tools for studying gene function and testing drugs. This type of technology can be used for the development of transgenic livestock and plants, to produce therapeutic proteins and antibodies on an industrial scale, and even to produce disease-resistant crops.
GeneCure’s lentiviral-based gene transfer technology can be used to efficient deliver the DNA of interest into embryonic stems cells of virtually any cell type including mammalian and non-mammalian cells. Thus, this technology can be used to generate transgenic animals, including those that have proven challenging using other methods.
Gene Silencing using siRNAs
Before a gene can be expressed as its protein product, the DNA encoding the gene is copied into a messenger RNA (mRNA) intermediate. It is this mRNA intermediate that is translated into the final protein product. Interfering with the mRNA intermediate is one method of silencing gene expression and is a powerful tool for genetic analysis in vivo and in vitro. Genes can be silenced through the use of small inhibitory RNAs (siRNAs). siRNAs are small molecules that play a crucial role in the destruction of RNA. siRNAs can be designed to target a specific mRNA and to turn off the expression of a specific gene of interest.
Viral vectors are commonly used to deliver siRNAs into cells or whole animals. GeneCure’s unique lentiviral-based gene transfer technology can be used to deliver siRNAs to mammalian and non-mammlian cells in vitro and in vivo. Even though use of siRNAs is still relatively new, its application has already allowed rapid evaluation of gene functions, and will most likely advance the development of innovative gene-based therapeutics. To date lentiviral-based vectors have been used to deliver stable and targeted siRNAs into primary mammalian cells, stem cells, and transgenic animals.
Stem Cell Manipulation
It has proven particularly challenging to genetically manipulate stem cells. Currently, onco-retroviruses are the most widely used gene transfer vectors. However, onco-retroviruses cannot efficiently deliver genes into stem cells. GeneCure’s lentiviral-based gene transfer technology offers unique biological properties that allow efficient and stable gene delivery into stem cells.
Animal models for human disease
Many human genetic and acquired diseases can be modeled by introducing a gene mutation or an entire gene into a mouse or other animal. While similar genetic manipulations can be achieved in tissue culture cells, expression of the gene in whole organisms provides for a much more comprehensive and physiologically relevant interpretation of the genes normal function. Animal models of human diseases are invaluable tools that provide both a better understanding of the disease and a model in which novel therapeutic approaches can be tested. GeneCure’s lentiviral-based gene transfer technology can be used to produce transgenic animals that model human diseases including diseases that affect non-dividing cells like Parkinson’s disease which affects neurons.
Gene Discovery
Although sequencing of the human genome has been completed, the physiological function of many genes remains to be elucidated. GeneCure’s lentiviral-based gene transfer technology can be used to deliver genes with unknown functions into mammalian cells in order to evaluate their functions. This technology can also be used to screen genes for therapeutic properties, with the aim of discovering novel therapeutic approaches to treat human diseases.
Reporter or marker gene expression in vitro and in vivo
Reporter or marker genes are often introduced into cells or whole organisms in order to track a specific cell population. They are used in countless research applications such as monitoring the spread of tumor cells in animal models of cancer. GeneCure’s lentiviral-based gene transfer technology allows efficient gene delivery into virtually any mammalian and non-mammalian cell type. This technology can be used to deliver a reporter or marker gene into both tissue culture cells and whole organisms.
HIT IT..!!!
http://www.empowernetwork.com/almostasecret.php?id=1210296
Hey ,I have a better option for earning online , faster money making and highly systematic system designed especially for you guys ,
click here if YOU: WANNA GET YOUR DEBTS OFF ..
DRIVE A FERRARI
LIVE IN A MANSION
ENJOY ON THE BEACHES OF THE WOLRD !!
HIT IT..!!!
http://www.empowernetwork.com/almostasecret.php?id=1210296
Hey ,I have a better option for earning online , faster money making and highly systematic system designed especially for you guys ,
click here if YOU: WANNA GET YOUR DEBTS OFF ..
DRIVE A FERRARI
LIVE IN A MANSION
ENJOY ON THE BEACHES OF THE WOLRD !!
.jpg)
.jpg)

 Watch DNA Travel Through The Conjugation Tube.
Watch DNA Travel Through The Conjugation Tube.

.jpg)

.gif)

 I
I